
__We must start our discussion with a look at the electromagnetic spectrum (EM), see the figure above.The visible portion of the EM spectrum is a very narrow band of wavelengths from about 400 to 700 nanometers (nm or 1×10-9meters). The area we are most concerned with is what is called near ultra violet (UV) or “actinic” light. This is light whose peak intensity is centered around a wavelength at approximately 420 nm. All radiative energy has an associated wavelength (λ) and and frequency (f).One is a function of the other: f=C/λ and λ=C/f, where C is the speed of light (3×108 meters per second, or m/s).
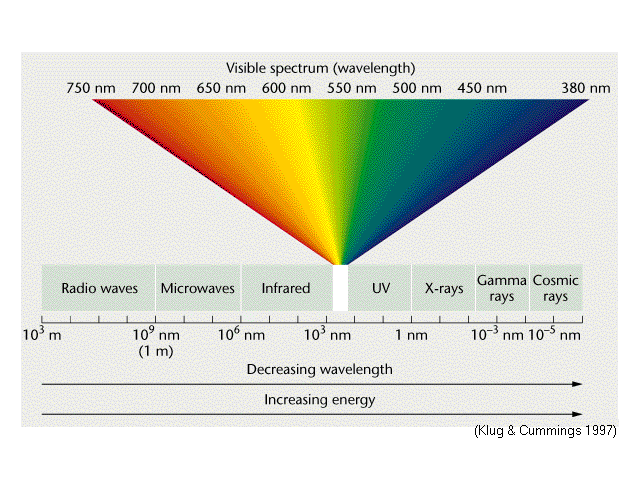
Viewing the figure above, wavelength is the distance in meters from one peak (or any other reference point on a wave) to the next peak (or next reference point) along the x-axis. Frequency is simply the number of times that wavelength occurs in one second and has an inverse relationship to wavelength. The higher the frequency, the shorter the wavelength and the higher the associated energy. Inversely, the longer the wavelength, the lower the frequency with associated lower energy (all other factors being equal). The y-axis is called the amplitude of the waveform.The term “actinic” is somewhat of a throwback to the early days of photography. Early films were not sensitive to red light and hence the term “safe lights” refers to dim red lights which were used in darkrooms during processing. Films of that era were sensitive to blue or “actinic” light, however. The term “actinic” is also used in many scientific papers on the subject of coral fluorescence. Referring again to the figure above, you see that this is in the deep blue, nearly violet portion of the spectrum.As all divers know, blue is the last color of the spectrum to be absorbed by water, hence near-actinic light is the light most sea creatures are exposed to, and that is also the light which is available for their photosynthesis and that which they have evolved under over the eons of time..Referring again to the figure of the electromagnetic spectrum above, note that as one moves to the right of the scale, the energy levels increase. Therefore UV light has more energy than red light. This will play an important role in our discussion below.Please also refer to the above figure whenever you see references to wavelength and frequency throughout this discussion.A “Full Spectrum Light” is a light source which emits all the wavelengths of the visible spectrum in the same proportions as natural sunlight.A lamp, LED, or bulb labeled “full spectrum” means that it emits light over the entire visible spectrum with a spectral output similar to that of the sun. This is what a standard white light underwater dive light (or torch) produces.
What we as divers need to have in order for the effect of fluorescence to occur and in order to be able to observe it are two things:
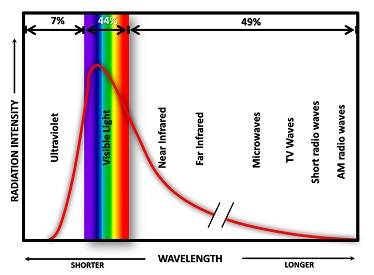
Above is a graph representing the output of the sun (solar irradiance). Note where the peak amplitude is – right there at the actinic band of wavelengths. As a side note, the numbers at the top of the figure represent the percentages of energy emitted by the sun at various wavelengths. About 7% is in the UV range, 44% is visible light and the remaining 49% is infrared and beyond. That last 49% is felt by you as warmth.
If you have any questions, comments or suggestions – PLEASE don’t hesitate to contact us.