
The Science Behind Light Fluorescence in Dive Photography
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. Fluorescence occurs when an orbital electron of a molecule, atom or nano-structure relaxes back to its ground state, thereby emitting a photon of light, after being excited to a higher quantum state by some type of energy. In our case, we are “hitting” an organism with higher energy light (relatively) in the near-actinic range, and lower energy light (relatively) in the green, yellow and red portion of the spectrum is being emitted. The actual color emitted is determined by how many quantum states the electron has “decayed” or relaxed back to.
The figure below represents this schematically.
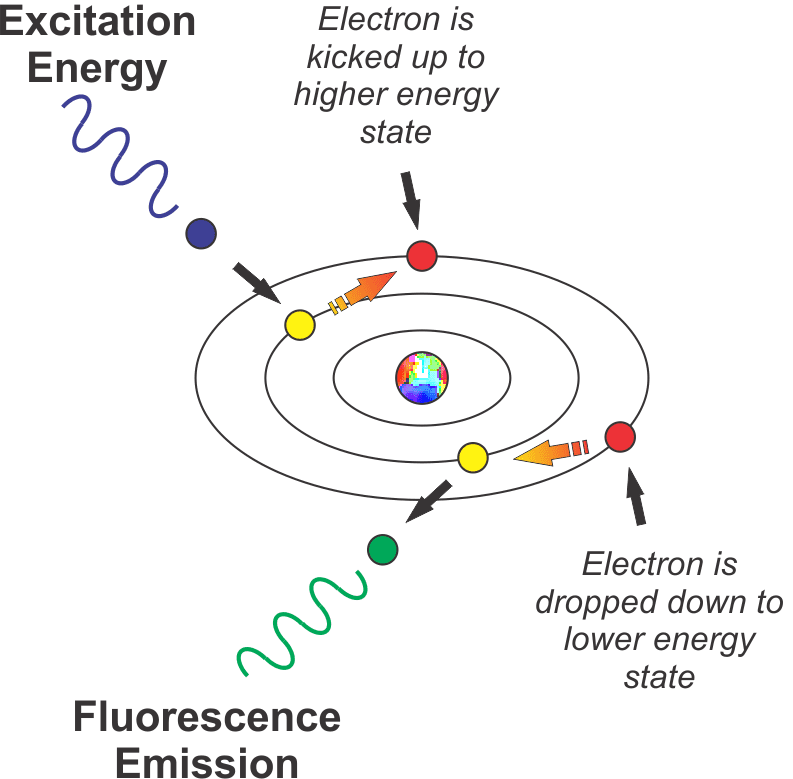
When energy (UV or violet/blue light in our example) strikes an atom, it knocks an electron up to a higher energy state. When the electron decays back to its normal state (usually instantly, after a few nanoseconds), it emits a photon of light (in the more visible, lower energy part of the spectrum in our example).
If you have any questions, comments or suggestions – PLEASE don’t hesitate to contact us.